Our Programs
Science
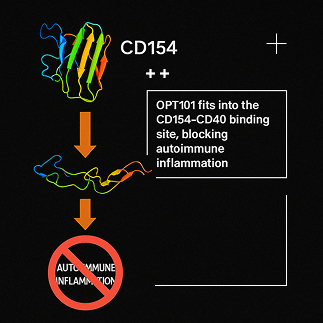
Elevated Th40 levels – T cells expressing CD40 – are strongly associated with autoimmune inflammation. CD40 binds with CD154, triggering a cascade of inflammatory signals. In most autoimmune diseases,
this cascade cannot be regulated back to healthy levels.
Op-T’s peptides are designed to regulate, not suppress, chronic inflammation by binding to CD40 on immune cells. By modulating CD40 signaling, our peptides help restore balance without shutting down the immune system. This prevents long-term tissue damage in vital organs.
Op-T’s Unique Approach
Op-T peptides control chronic inflammation without suppressing immunity. Unlike current treatments that blunt the immune system entirely – often leading to serious side effects – Op-T’s approach preserves normal immune defense while targeting only the dysregulated components.
Pipeline
Op-T has 3 human drug candidates, plus 1 animal drug candidate, in the following stages of development
Pre-Clinical Results in NOD Mice
In late-stage, pre- diabetic NOD mice, peptide treatment significantly improved islet function, restoring systemic glucose control and maintaining normal insulin levels.
In diabetic mice with blood glucose >250 mg/dL, OPT101 restored glucose to normal levels in 57% of treated mice.
Prevention and Delayed Diabetes Onset in Mice
Type 1 Diabetes
Op-T has developed a therapeutic peptide to control the inflammatory T cells at the core of autoimmune diseases, including type 1 diabetes (T1D) , Multiple Sclerosis (MS), and others.
Across multiple studies in humans, dogs with diabetes, and the mouse model of diabetes, Op-T’s lead peptide, known as OPT101, restored immune balance during T1D, improved glucose tolerance and increased insulin production.
In a Phase 1b clinical trial in humans with T1D, two subjects with undetectable C-peptide levels at baseline showed detectable levels after 6 weeks of treatment with OPT101. Subjects with residual C-peptide also experienced increases in C-peptide levels.
Op-T is preparing to commence a Phase 2a study in May of 2025 to evaluate OPT101 in a larger T1D population over a 12-month treatment period.
Most current treatments for T1D are monoclonal antibodies, which:
- Are difficult and expensive to produce
- Can cause unintended problems including kidney failure
- Don’t address the root autoimmune issue
- Often stop working over time
Insulin, no matter how it is delivered, is not a treatment – it is a therapy
Sepsis
Sepsis is a serious, life-threatening global health problem. Worldwide incidence is 49 million people with 11 million deaths annually. In the United States, 1.7 million people are affected, with 350,000 people dying annually.
Despite being a leading cause of death in hospitalized patients, specific treatments are non-existent despite advances in supportive care.
At present, there are limited treatment options for the primary cause of the severe pathology, immune dysregulation, leading to outcomes such as Cytokine Storm and Systemic Inflammatory Response Syndrome (SIRS).
Current therapies in sepsis include antibiotics for infectious agents, steroids to treat long-term effects, and single target monoclonal antibodies for individual inflammatory cytokines.
Op-T is preparing to commence a Phase 1b study in May of 2025, evaluating OPT101 in 18 subjects with sepsis.
Multiple Sclerosis
More than 1 million people in the US and 2.8 million worldwide suffer from MS while the incidence rate continues to increase.
There currently are 25 FDA approved disease modulating therapies (DMT) for MS, with each of these drugs modulating a component of the immune system. While DMTs reduce relapse frequency and MRI lesion burden, most do not halt long-term disease progression, especially in progressive MS.
Op-T has developed a series of peptides that target CD40 on adaptive and innate immune cells. In mouse models of MS, Op-T peptides have significantly delayed disease onset and improved symptoms.
Op-T therapeutics are not monoclonal antibodies.
Op-T Veterinary
Op-T is developing OPT501, a veterinary version of its peptide, for the treatment of canine diabetes.
In treated dogs, OPT501 significantly reduced blood and urine glucose, eliminated ketonuria, and improved time-in-range (blood glucose between 80-160 mg/dL). No glucose was detected in the urine in most cases.
Treated dogs also showed reductions infructosavine (the canine equivalent of HbA1c), lower blood glucose, decreased insulin use, and improved metabolic markers.
After 8 weeks of treatment, dogs had increased C-peptide levels, and no negative impacts on white or red blood cell counts. Liver, kidney, and cholesterol profiles remained normal or improved. OPT501 improved glycemic control in diabetic dogs without inducing immune suppression or hematologic toxicity.